Efficient synthesis of pyrene-1-carbothioamides and carboxamides. Tunable solid-state fluorescence of pyrene-1-carboxamides
Date
2014-10-23Author
Wrona-Piotrowicz, Anna
Zakrzewski, Janusz
Métivier, Rémi
Brosseau, Arnaud
Makal, Anna
Woźniak, Krzysztof
Metadata
Show full item recordAbstract
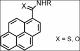
Pyrene reacts with potassium thiocyanate and organic isothiocyanates in the presence of
trifluoromethanesulfonic acid to afford primary and secondary pyrene-1-carbothioamides in high yields.
These compounds were efficiently oxidatively desulfurized with Oxone® to the corresponding
carboxamides. The amides display solid-state fluorescence with quantum efficiencies up to 62%,
originating from monomers, aggregates (such as preformed dimers), and/or excimers, depending on the
substituent at the nitrogen atom. Single crystal X-ray diffraction characterization of one highly emissive
compound supports this assumption.
Collections
The following license files are associated with this item:

